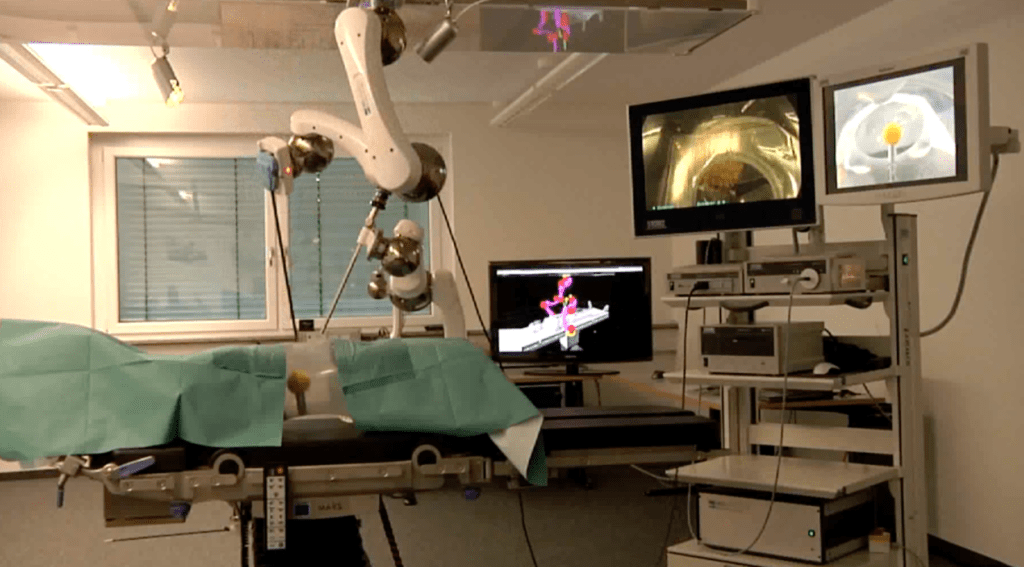
AVRA Medical Robotics has stabilized its finances as it continues developing a fully autonomous surgical system and submitting it for regulatory approval.
Source: AVRA Medical Robotics
AVRA Medical Robotics Inc. this week announced that it has paid off nearly all of its external corporate debt, including accrued interest.
The Orlando, Fla.–based company said it cleared its debt with the exception of loans from its CEO and a loan note for $25,000, which is due on Dec. 31, 2020. One note of $25,000 is being converted into shares at $1.50 per share, with the remainder being repaid in full with accrued interest, said AVRA.
“The repayment of all this company debt provides further stability to the company’s finances, allowing us to focus on our medical software procedure program and the development of our Autonomous Robotics Surgical System,” stated Barry Cohen, chairman and CEO of AVRA Medical Robotics. “The potential of our robotic systems is to perform operations with greater precision than human hands are capable of.”
The process of raising funds while developing medical robotics products and submitting them to the U.S. Food and Drug Administration can be challenging. For example, Titan Medical Inc. this week acknowledged that it needs to raise $85 million for its Sport robot-assisted surgery device.
AVRA continues development, regulatory approval process
The company said it is developing a fully autonomous system to “robotize” a wide range of surgical procedures. Most surgical robots today are designed to assist humans conducting the procedures.
AVRA has a research agreement with the University of Central Florida. Last fall, it appointed Dr. Vipu Patel and Dr. Eytan Pollak to chair its Medical Advisory Board and Scientific Advisory Board, respectively.
AVRA is currently working on a treatment-independent, precision-guidance system, with an initial focus on noninvasive skin-resurfacing aesthetic procedures.
Last fall, AVRA announced that it had begun the process of submitting its fully autonomous robot to the FDA for approval. At the time, AVRA said it had plans to pursue simultaneous approvals in the U.S., Canada, Europe, Australia, Japan and Brazil. The system already has CE Mark approval from European regulators, according to the company.
For more coverage of medical devices and surgical robotics, visit Robotics Business Review‘s sibling sites, MassDevice and The Robot Report.