In recent years, zebrafish (Danio rerio) have risen in popularity as useful predictive model organisms in several research fields. These tropical freshwater minnows share about 70% of their genes with humans, so studies devoted to mapping critical pathways and characterizing phenotypes in zebrafish should yield information and insights that advance the understanding and treatment of human disorders and diseases such as diabetes, heart disease, and cancer.
Compared to traditional rodent model organisms, zebrafish offer several research advantages. Their minimal environmental requirements, high offspring rates, and shoaling behaviors make zebrafish easier and less expensive to maintain than mammalian models, while the small size of zebrafish embryos enables use of multiwell plates and in vivo high-throughput, high-content screening with minimal amounts of reagents and drug compounds. It is relatively easy to introduce genetic changes to zebrafish populations, as they absorb chemical mutagens into their systems through the surrounding aquatic habitat, whereas mammalian models require individual, labor-intensive topical, oral, or intravenous administration methods. Zebrafish embryos develop externally without the need for stress-inducing invasive techniques or maternal sacrifice, and also develop rapidly such that organogenesis is completed within 48 hours postfertilization, reducing overall project time.
Perhaps the most unique and significant advantage of the zebrafish model is the optical transparency of embryos and larvae, which makes it possible to directly monitor natural development and processes, or the influence of a genetic manipulation or compound treatment, in cells, tissues, and whole organisms using noninvasive imaging techniques.
Automated quantitative zebrafish imaging
The Lionheart™ FX Automated Microscope is a digital widefield microscope that is suitable for both endpoint and kinetic imaging of zebrafish embryos in microplates. Wide assay flexibility is enabled through brightfield, color brightfield, phase contrast, and fluorescence imaging channels; environmental controls in the imaging chamber ensure optimal conditions for long-term kinetic imaging. Integrated Gen5™ Microplate Reader and Imager Software controls the automated image capture, processing, and analysis, including dual masking, object-based spot counting, graphic annotation, and video production. Here, we demonstrate the quantitative image analysis capabilities the Lionheart FX provides when it is used to assess drug-induced phenotype changes to zebrafish embryos.
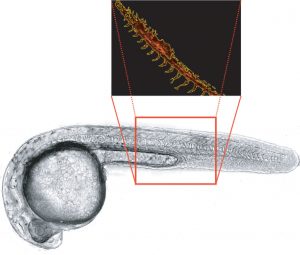
As zebrafish embryos develop, the blood vessel pattern of their circulatory system is highly characteristic and easily visualized microscopically. This makes zebrafish a good primary screen for compounds that impact angiogenesis, or the formation of new blood vessels, in other vertebrates, including humans. Factors that promote or inhibit angiogenesis could impact research areas ranging from heart disease to tissue regeneration and cancer biology. Vascular endothelial growth factor (VEGF) regulates angiogenesis and is expressed in the vicinity of sprouting vessels, while its tyrosine kinase receptor, VEGFR2 (also known as kdrl) is expressed in endothelial cells.
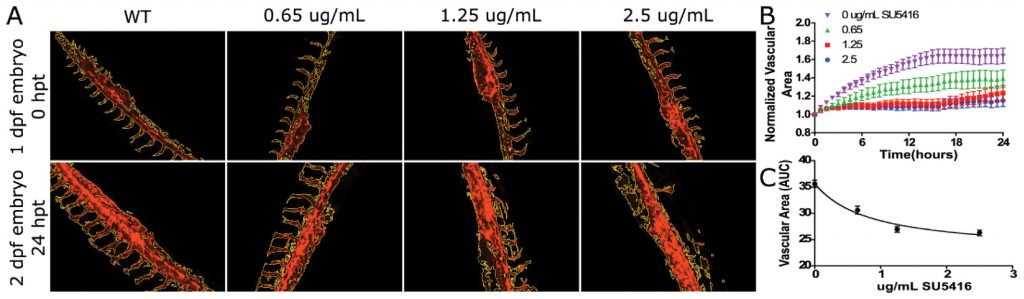
In this example, transgenic Tg(kdrl:mcherry) zebrafish embryos expressing red fluorescence in blood vessels are used to analyze vascular development over 24 hours of treatment with the VEGF inhibitor SU5416. A beacon is used to identify the zebrafish embryo region of interest (Figure 1), and time-lapse fluorescence images of control and treated embryos are automatically captured using the Lionheart FX. The integrated software’s masking function is used to precisely determine vasculature area changes over time in postprocessed images, demonstrating that zebrafish embryo vascular growth is repressed by the inhibitor in a dose- dependent manner (Figure 2).
Zebrafish embryos possess the ability to regenerate tissue in response to injury. In one of the many overlapping mechanisms that occur in response to a wound, neutrophils appear to protect the site from foreign invaders. Characterizing this and other underlying wound healing actions in zebrafish can translate into accelerated tissue repair and disease treatments in humans and other vertebrates.
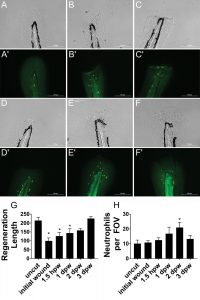
Here, we use transgenic zebrafish embryos to monitor wound healing over time following transection of the caudal fin, which consists of two epithelial layers. The embryos are treated with a microtubule-destabilizing compound, nocodazole, prior to and after wound creation to demonstrate its effect on neutrophils. At various stages over three days, the whole zebrafish embryos are fixed, permeabilized, incubated with primary antibody and fluorescently labeled secondary antibody, and mounted for imaging. The Gen5 software is used in the Lionheart FX, and a beacon is placed around the caudal tail region prior to brightfield and fluorescent imaging. After imaging and postimaging processing, object masks are automatically placed around the tail and notochord to determine regeneration length and to count immune cells at the various timepoints (Figure 3). Using this data, we determine that zebrafish embryos regenerate almost completely by the third day, postwounding.
In summary, zebrafish demonstrate great utility in yielding insights that can benefit other vertebrate species, including humans, with less expense and simpler workflows compared to traditional mammalian models. When coupled with automated digital imaging and quantitative image analysis using the Lionheart FX, the zebrafish provides a compelling whole-organism model of high physiological relevance for drug discovery applications.
Peter Banks, PhD (banksp@biotek.com), serves as scientific director at BioTek Instruments, and Sarah Beckman, PhD, is a medical writer and former principal scientist at the company.