What You Should Know:
– LexaGene’s closes CAD$13 million funding round as it
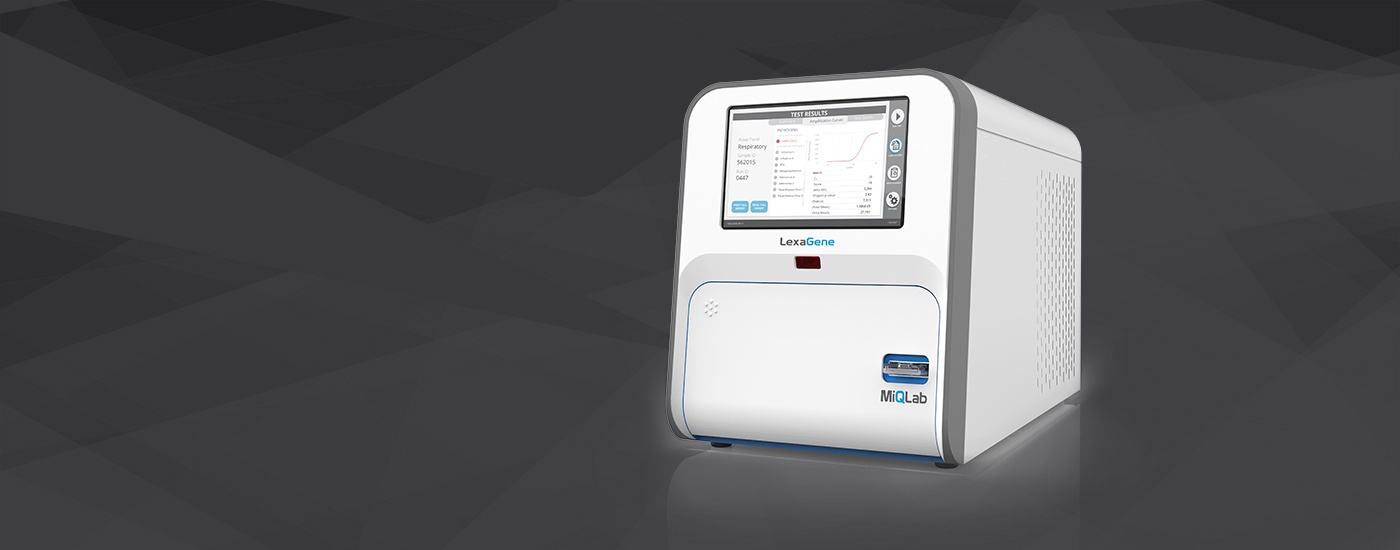
prepares to launch its gold standard diagnostic device called MiQLab that
applies a proprietary RT-qPCR-based method to analyze each sample’s contents
for up to 27 different pathogens in approximately one hour.
– MiQLab is uniquely open-access, which allows users to
customize their tests and create new tests easily. In the case where a new test
was needed because of a mutation, the open-access feature allows new tests to
be deployed quickly on LexaGene’s MiQLab system.
LexaGene Holdings Inc., a molecular diagnostics company that develops genetic analyzers for pathogen detection and other molecular markers for on-site rapid testing has raised CAD$13.29 million in funding.
Automated, Genetic Analyzer Designed for Rapid, Point-of-Need
Testing
LexaGene has developed a gold standard diagnostic device
called MiQLab that applies a
proprietary RT-qPCR-based method to analyze each sample’s contents for up to 27
different pathogens in approximately one hour. MiQLab is uniquely open-access,
which allows users to customize their tests and create new tests easily. In the
case where a new test was needed because of a mutation, the open-access feature
allows new tests to be deployed quickly on LexaGene’s MiQLab system.
Scientists who have custom testing needs make up the
open-access market, and they currently spend hours a day to manually perform
PCR testing. These same individuals will now have the opportunity to save hours
a day by having MiQLab automate the processing of their own tests.
LexaGene has submitted a preliminary plan for COVID-19
testing to the FDA and is still in conversations with the agency regarding the
proposed studies for this novel technology. As is standard practice, until the
FDA grants LexaGene’s instrument EUA for COVID-19 testing, all work using
LexaGene instruments is classified as Research Use Only and cannot be used for
human clinical diagnostics.